Which Drawing Below Best Represents Hydrogen Bonding Methanol, Ch3oh?
Hydrogen Bonding
- Page ID
- 1660
A hydrogen bond is an intermolecular force (IMF) that forms a special type of dipole-dipole allure when a hydrogen atom bonded to a strongly electronegative atom exists in the vicinity of some other electronegative cantlet with a lone pair of electrons. Intermolecular forces (IMFs) occur betwixt molecules. Other examples include ordinary dipole-dipole interactions and dispersion forces. Hydrogen bonds are are by and large stronger than ordinary dipole-dipole and dispersion forces, but weaker than true covalent and ionic bonds.
The testify for hydrogen bonding
Many elements grade compounds with hydrogen. If you lot plot the humid points of the compounds of the grouping 14 elements with hydrogen, yous observe that the boiling points increase as you lot go downward the group.
The increase in boiling point happens because the molecules are getting larger with more electrons, and so van der Waals dispersion forces go greater. If yous echo this exercise with the compounds of the elements in groups 15, 16, and 17 with hydrogen, something odd happens.
Although the same reasoning applies for grouping 4 of the periodic table, the humid point of the compound of hydrogen with the kickoff element in each grouping is abnormally high. In the cases of \(NH_3\), \(H_2O\) and \(HF\) there must be some boosted intermolecular forces of allure, requiring significantly more heat free energy to break the IMFs. These relatively powerful intermolecular forces are described as hydrogen bonds.
Origin of Hydrogen Bonding
The molecules capable of hydrogen bonding include the post-obit:
Discover that in each of these molecules:
- The hydrogen is attached directly to a highly electronegative atoms, causing the hydrogen to acquire a highly positive accuse.
- Each of the highly electronegative atoms attains a high negative charge and has at least one "active" alone pair. Lone pairs at the 2-level accept electrons contained in a relatively small book of space, resulting in a high negative accuse density. Lone pairs at higher levels are more than diffuse and, resulting in a lower charge density and lower affinity for positive accuse.
If you are not familiar with electronegativity, yous should follow this link before you proceed.
Consider two water molecules coming shut together.
The \(\delta^+\) hydrogen is so strongly attracted to the lone pair that it is nearly equally if you were kickoff to form a co-ordinate (dative covalent) bond. Information technology doesn't go that far, but the attraction is significantly stronger than an ordinary dipole-dipole interaction. Hydrogen bonds accept about a tenth of the strength of an average covalent bond, and are constantly cleaved and reformed in liquid water. If you lot liken the covalent bond between the oxygen and hydrogen to a stable union, the hydrogen bond has "just good friends" status.
Water is an ideal example of hydrogen bonding. Notice that each h2o molecule can potentially grade 4 hydrogen bonds with surrounding water molecules: two with the hydrogen atoms and two with the with the oxygen atoms. There are exactly the correct numbers of \(\delta^+\) hydrogens and alone pairs for every one of them to be involved in hydrogen bonding.
This is why the boiling signal of water is college than that of ammonia or hydrogen fluoride. In the instance of ammonia, the amount of hydrogen bonding is express by the fact that each nitrogen only has one lone pair. In a group of ammonia molecules, in that location are not plenty lone pairs to go around to satisfy all the hydrogens. In hydrogen fluoride, the problem is a shortage of hydrogens. In water, two hydrogen bonds and two lone pairs allow germination of hydrogen bond interactions in a lattice of water molecules. Water is thus considered an ideal hydrogen bonded arrangement.
More complex examples of hydrogen bonding
The hydration of negative ions
When an ionic substance dissolves in water, water molecules cluster around the separated ions. This process is called hydration. H2o oft attaches to positive ions by according (dative covalent) bonds. Information technology bonds to negative ions using hydrogen bonds.
If you are interested in the bonding in hydrated positive ions, you could follow this link to according (dative covalent) bonding.
The diagram shows the potential hydrogen bonds formed with a chloride ion, Cl-. Although the alone pairs in the chloride ion are at the 3-level and would non normally be active plenty to form hydrogen bonds, they are fabricated more than attractive past the full negative charge on the chlorine in this case.
However complicated the negative ion, there volition e'er be solitary pairs that the hydrogen atoms from the water molecules can hydrogen bond to.
Hydrogen bonding in alcohols
An alcohol is an organic molecule containing an -OH grouping. Any molecule which has a hydrogen atom attached straight to an oxygen or a nitrogen is capable of hydrogen bonding. Hydrogen bonds also occur when hydrogen is bonded to fluorine, but the HF group does not announced in other molecules. Molecules with hydrogen bonds will e'er have college humid points than similarly sized molecules which don't accept an an -O-H or an -N-H group. The hydrogen bonding makes the molecules "stickier," such that more heat (energy) is required to dissever them. This phenomenon can be used to analyze boiling bespeak of dissimilar molecules, defined every bit the temperate at which a phase change from liquid to gas occurs.
Ethanol, \(\ce{CH3CH2-O-H}\), and methoxymethane, \(\ce{CH3-O-CH3}\), both accept the same molecular formula, \(\ce{C2H6O}\).
They have the aforementioned number of electrons, and a like length. The van der Waals attractions (both dispersion forces and dipole-dipole attractions) in each will exist like. However, ethanol has a hydrogen atom attached directly to an oxygen; hither the oxygen notwithstanding has two lonely pairs like a water molecule. Hydrogen bonding can occur betwixt ethanol molecules, although not as finer as in water. The hydrogen bonding is express by the fact that there is only one hydrogen in each ethanol molecule with sufficient + accuse.
In methoxymethane, the lone pairs on the oxygen are nonetheless there, but the hydrogens are not sufficiently + for hydrogen bonds to form. Except in some rather unusual cases, the hydrogen atom has to be attached directly to the very electronegative chemical element for hydrogen bonding to occur. The boiling points of ethanol and methoxymethane show the dramatic effect that the hydrogen bonding has on the stickiness of the ethanol molecules:
| ethanol (with hydrogen bonding) | 78.five°C |
| methoxymethane (without hydrogen bonding) | -24.8°C |
The hydrogen bonding in the ethanol has lifted its boiling signal about 100°C. It is important to realize that hydrogen bonding exists in addition to van der Waals attractions. For example, all the following molecules incorporate the same number of electrons, and the showtime 2 have similar chain lengths. The higher boiling point of the butan-1-ol is due to the additional hydrogen bonding.
Comparing the two alcohols (containing -OH groups), both boiling points are high considering of the boosted hydrogen bonding; however, the values are non the same. The boiling signal of the 2-methylpropan-1-ol isn't as high every bit the butan-one-ol considering the branching in the molecule makes the van der Waals attractions less constructive than in the longer butan-ane-ol.
Hydrogen bonding in organic molecules containing nitrogen
Hydrogen bonding also occurs in organic molecules containing Northward-H groups; recall the hydrogen bonds that occur with ammonia. Examples range from simple molecules like CH3NHtwo (methylamine) to big molecules like proteins and DNA. The two strands of the famous double helix in DNA are held together by hydrogen bonds between hydrogen atoms attached to nitrogen on ane strand, and solitary pairs on some other nitrogen or an oxygen on the other one.
Donors and Acceptors
In order for a hydrogen bond to occur there must be both a hydrogen donor and an acceptor present. The donor in a hydrogen bond is commonly a strongly electronegative cantlet such equally N, O, or F that is covalently bonded to a hydrogen bail.
The hydrogen acceptor is an electronegative atom of a neighboring molecule or ion that contains a lone pair that participates in the hydrogen bond.
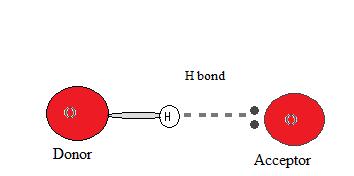
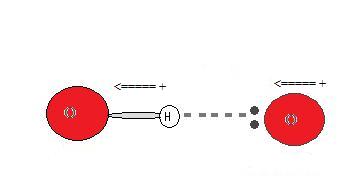
Why does a hydrogen bond occur?
Since the hydrogen donor (Northward, O, or F) is strongly electronegative, it pulls the covalently bonded electron pair closer to its nucleus, and away from the hydrogen atom. The hydrogen atom is then left with a partial positive accuse, creating a dipole-dipole attraction betwixt the hydrogen atom bonded to the donor and the lone electron pair of the acceptor. This results in a hydrogen bond.(see Interactions Between Molecules With Permanent Dipoles)
Types of hydrogen bonds
Although hydrogen bonds are well-known as a blazon of Imf, these bonds tin also occur within a single molecule, betwixt two identical molecules, or between 2 different molecules.
Intramolecular hydrogen bonds
Intramolecular hydrogen bonds are those which occur within i unmarried molecule. This occurs when two functional groups of a molecule can form hydrogen bonds with each other. In order for this to happen, both a hydrogen donor a hydrogen acceptor must be nowadays inside one molecule, and they must be within close proximity of each other in the molecule. For example, intramolecular hydrogen bonding occurs in ethylene glycol (C2H4(OH)2) betwixt its two hydroxyl groups due to the molecular geometry.
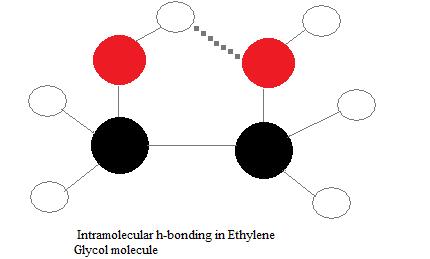
Intermolecular hydrogen bonds
Intermolecular hydrogen bonds occur betwixt separate molecules in a substance. They can occur betwixt any number of like or unlike molecules equally long as hydrogen donors and acceptors are present in positions where they tin collaborate with 1 another. For example, intermolecular hydrogen bonds can occur betwixt NHiii molecules lonely, betwixt HtwoO molecules lonely, or betwixt NH3 and H2O molecules.
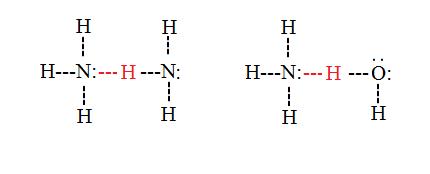
Properties and effects of hydrogen bonds
On Boiling Betoken
When we consider the boiling points of molecules, nosotros usually expect molecules with larger molar masses to have higher normal humid points than molecules with smaller molar masses. This, without taking hydrogen bonds into account, is due to greater dispersion forces (see Interactions Betwixt Nonpolar Molecules). Larger molecules accept more space for electron distribution and thus more than possibilities for an instantaneous dipole moment. However, when we consider the table below, nosotros see that this is not e'er the example.
| Compound | Molar Mass | Normal Humid Signal |
|---|---|---|
| \(H_2O\) | 18 k/mol | 373 M |
| \(HF\) | 20 g/mol | 292.5 M |
| \(NH_3\) | 17 yard/mol | 239.viii K |
| \(H_2S\) | 34 g/mol | 212.9 K |
| \(HCl\) | 36.4 m/mol | 197.9 K |
| \(PH_3\) | 34 g/mol | 185.2 Thou |
We see that HtwoO, HF, and NH3 each have college boiling points than the same compound formed betwixt hydrogen and the next chemical element moving down its corresponding grouping, indicating that the erstwhile take greater intermolecular forces. This is because H2O, HF, and NH3 all showroom hydrogen bonding, whereas the others practise not. Furthermore, \(H_2O\) has a smaller molar mass than HF but partakes in more hydrogen bonds per molecule, so its boiling signal is college.
On Viscosity
The aforementioned issue that is seen on humid bespeak as a upshot of hydrogen bonding tin can also be observed in the viscosity of certain substances. Substances capable of forming hydrogen bonds tend to have a higher viscosity than those that practise not for hydrogen bonds. Generally, substances that take the possibility for multiple hydrogen bonds exhibit fifty-fifty higher viscosities.
Factors preventing Hydrogen bonding
Electronegativity
Hydrogen bonding cannot occur without significant electronegativity differences between hydrogen and the atom information technology is bonded to. Thus, we see molecules such as PH3, which no not partake in hydrogen bonding. PHiii exhibits a trigonal pyramidal molecular geometry like that of ammonia, but unlike NH3 information technology cannot hydrogen bond. This is due to the similarity in the electronegativities of phosphorous and hydrogen. Both atoms accept an electronegativity of 2.ane, and thus, no dipole moment occurs. This prevents the hydrogen bonding from acquiring the partial positive accuse needed to hydrogen bail with the solitary electron pair in another molecule. (run across Polarizability)
Cantlet Size
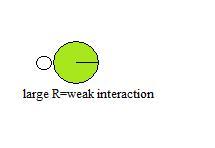
The size of donors and acceptors can too effect the ability to hydrogen bail. This can account for the relatively low ability of Cl to form hydrogen bonds. When the radii of two atoms differ greatly or are large, their nuclei cannot attain close proximity when they interact, resulting in a weak interaction.
Hydrogen Bonding in Nature
Hydrogen bonding plays a crucial function in many biological processes and can account for many natural phenomena such every bit the Unusual properties of Water. In improver to beingness nowadays in water, hydrogen bonding is also important in the h2o send organization of plants, secondary and tertiary poly peptide construction, and DNA base pairing.
Plants
The cohesion-adhesion theory of transport in vascular plants uses hydrogen bonding to explicate many key components of water movement through the plant's xylem and other vessels. Within a vessel, water molecules hydrogen bond not only to each other, but likewise to the cellulose concatenation which comprises the wall of plant cells. Since the vessel is relatively small, the attraction of the h2o to the cellulose wall creates a sort of capillary tube that allows for capillary activeness. This mechanism allows plants to pull water upwardly into their roots. Furthermore, hydrogen bonding can create a long chain of water molecules which tin can overcome the force of gravity and travel up to the high altitudes of leaves.
Proteins
Hydrogen bonding is present abundantly in the secondary structure of proteins, and also sparingly in 3rd conformation. The secondary structure of a protein involves interactions (mainly hydrogen bonds) between neighboring polypeptide backbones which incorporate Nitrogen-Hydrogen bonded pairs and oxygen atoms. Since both North and O are strongly electronegative, the hydrogen atoms bonded to nitrogen in i polypeptide courage tin hydrogen bond to the oxygen atoms in another chain and visa-versa. Though they are relatively weak, these bonds offer substantial stability to secondary protein structure because they repeat many times and work collectively.
In tertiary protein structure, interactions are primarily betwixt functional R groups of a polypeptide chain; ane such interaction is called a hydrophobic interaction. These interactions occur because of hydrogen bonding betwixt water molecules around the hydrophobe that further reinforces poly peptide conformation.
References
- Brown, et al. Chemistry:The Primal Science. 11th ed. Upper Saddle River, New Jersey: Pearson/Prentice Hall, 2008.
- Chang, Raymond. General Chemistry:The Essential Concepts. 3rd ed. New York: Mcgraw Hill, 2003
- Petrucci, et al. General Chemistry: Principles & Modern Applications. ninth ed. Upper Saddle River, New Jersey: Pearson/Prentice Hall, 2007.
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_%28Physical_and_Theoretical_Chemistry%29/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Intermolecular_Forces/Specific_Interactions/Hydrogen_Bonding
Posted by: hamiltonwathre.blogspot.com


0 Response to "Which Drawing Below Best Represents Hydrogen Bonding Methanol, Ch3oh?"
Post a Comment